Citation
Brendan Sullivan, Rick Archibald, Patricia S Langan, Holger Dobbek, Martin Bommer, Robert L McFeeters, Leighton Coates, Xiaoping P Wang, Franz Gallmeier, John M Carpenter, Vickie Lynch, Paul Langan, "Improving the accuracy and resolution of neutron crystallographic data by three-dimensional profile fitting of Bragg peaks in reciprocal space", Acta Crystallographica Section D: Structural Biology, 74(11), 2018
Abstract
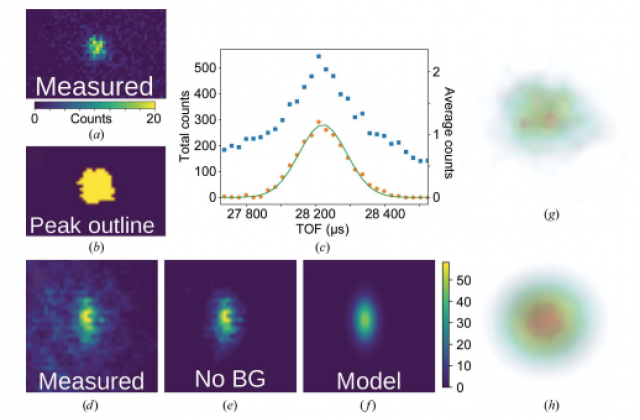
Neutron crystallography is a powerful technique for directly visualizing the locations of H atoms in biological macromolecules. This information has provided key new insights into enzyme mechanisms, ligand binding and hydration. However, despite the importance of this information, the application of neutron crystallography in biology has been limited by the relatively low flux of available neutron beams and the large incoherent neutron scattering from hydrogen, both of which contribute to weak diffraction data with relatively low signal-to-background ratios. A method has been developed to fit weak data based on three-dimensional profile fitting of Bragg peaks in reciprocal space by an Ikeda–Carpenter function with a bivariate Gaussian. When applied to data collected from three different proteins, three-dimensional profile fitting yields intensities with higher correlation coefficients (CC1/2) at high resolutions, decreased Rfree factors, extended resolutions and improved nuclear density maps. Importantly, additional features are revealed in nuclear density maps that may provide additional scientific information. These results suggest that three-dimensional profile fitting will help to extend the capabilities of neutron macromolecular crystallography
Read PublicationLast Updated: May 28, 2020 - 4:05 pm